
is joining VYGON

PEROUSE MEDICAL becomes the Cardio-Vascular and Adult Long term Vascular Access specialist within VYGON’s group
Interview with Dr Aline Steghens, anaesthetist, Dr Maxime Tardieu, radiologist and Malka Debbiche, Imaging Department health manager, Lyon-Sud Hospital Centre
In recent years, requirements for implantable ports (IP) have increased considerably. Implantation has been simplified by ultrasound-guided percutaneous insertion and technological advances have allowed new uses to be made for them, particularly in radiology (high pressure ports).
[…] We have been implanting IP in the radiology department since 2010. Previously this was done only by surgeons but with the very large increasing demands in the hospital combined with advances in implantation techniques, percutaneous ultrasound-guided. we have extended the implantation procedures for IP to radiologists. […]
[…] Percutaneous positioning with tunnelling avoids surgical incisions and ultrasound guiding visualises the anatomical structures (veins, arteries, pleura etc) and the needle, in order to avoid the risk of haematoma, arterial puncture, and pneumothorax and the number of puncture attempts. Ultrasound guidance which is part of the expert recommendations implanting a venous catheter or other vascular device’ which the HAS (French National Health Authority) published at the end of 2010. Ultrasound guiding reduces the number of punctures and as a result the risk of infections and thrombosis. […]
[…] For radiological procedures involving injection of contrast medium, the IP avoids the use of peripheral venous lines and gains time. The development of high pressure devices allows the port to be used for some procedures such as CT angiography, particularly coronary CT angiography, investigation for pulmonary embolism, tumour perfusion imaging, CVA etc […]
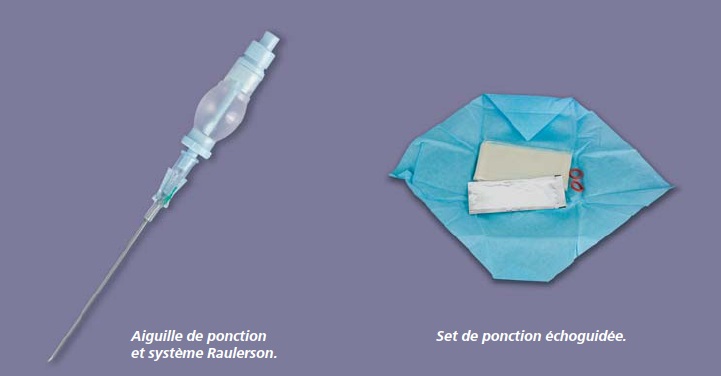 […] In general, PEROUSE MEDICAL offers high quality, well-designed materials to implant ports: their connection rings are very easy to use with identifiers facilitating fitting them: the tunneller is high performance, the needle is well bevelled, the guide is of good quality and the ovoid port is easy to insert. These materials are manufactured in France. This manufacturer also has a manufacturing site in Lyons very close to us. It has shown itself to be very responsive in designing specific kits depending on an establishment’s needs. In terms of the new POLYSITE®Echo ultrasound guidance kit, I have not yet had the opportunity to use it although I find the concept interesting. It is a complete kit including the ultrasound gel and ultrasound probe sheath. In particular it has a small chamber fitted with a non-return valve which fits on to the puncture needle and replaces the syringe to check for venous reflux. After the puncture we compress the chamber. Once the needle cutting edge is in the vein it fills with blood showing that the needle is correctly positioned in the vein. All that we need to do then is to place the guide through the needle holder set and remove the needle and chamber as one unit. This system therefore removes the stage of disconnecting the syringe from the needle and as a result the risk of gas embolism or secondary movement of the needle.
[…] In general, PEROUSE MEDICAL offers high quality, well-designed materials to implant ports: their connection rings are very easy to use with identifiers facilitating fitting them: the tunneller is high performance, the needle is well bevelled, the guide is of good quality and the ovoid port is easy to insert. These materials are manufactured in France. This manufacturer also has a manufacturing site in Lyons very close to us. It has shown itself to be very responsive in designing specific kits depending on an establishment’s needs. In terms of the new POLYSITE®Echo ultrasound guidance kit, I have not yet had the opportunity to use it although I find the concept interesting. It is a complete kit including the ultrasound gel and ultrasound probe sheath. In particular it has a small chamber fitted with a non-return valve which fits on to the puncture needle and replaces the syringe to check for venous reflux. After the puncture we compress the chamber. Once the needle cutting edge is in the vein it fills with blood showing that the needle is correctly positioned in the vein. All that we need to do then is to place the guide through the needle holder set and remove the needle and chamber as one unit. This system therefore removes the stage of disconnecting the syringe from the needle and as a result the risk of gas embolism or secondary movement of the needle.
Lire l'article complet.